Some devices are over-inflating, and some have been linked to acute pancreatitis, agency says
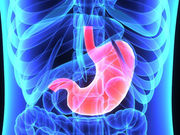
MONDAY, Feb. 13, 2017 (HealthDay News) — Fluid-filled intragastric balloons used to treat obesity have been linked to two different types of adverse events, according to the U.S. Food and Drug Administration.
Two types of fluid-filled balloon systems — the ReShape Integrated Dual Balloon System and the Orbera Intragastric Balloon System — were approved by the FDA in 2015. In a recent warning sent to health care providers, the FDA said it has received multiple reports of complications associated with the two balloon systems.
One adverse event reported is spontaneous hyperinflation resulting in the need for premature removal of the balloons. The second adverse event reported is development of acute pancreatitis, also resulting in need for device removal. The report notes acute pancreatitis developed in several patients due to the compression of gastrointestinal structures, and the condition can occur as soon as three days after implantation.
The FDA letter recommends that health care providers “closely monitor patients with these devices for these adverse events, and to submit reports to help us better understand any complications from the use of these obesity treatment devices.” The agency said it is working with the manufacturers to better understand the problems caused by the fluid-filled balloon systems. The FDA said it will provide more information as the investigation continues. No problems have been reported with another type of balloon system used to treat obesity — the Obalon system — which uses only air, according to the FDA.
Copyright © 2017 HealthDay. All rights reserved.