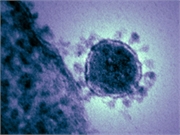
Trial to be conducted in the central Chinese city of Wuhan, the epicenter of the outbreak
MONDAY, Feb. 3, 2020 (HealthDay News) — A clinical trial to test an experimental antiviral drug’s effectiveness against the new coronavirus will be conducted in China as it battles a coronavirus outbreak there.
The drug Remdesivir — created to fight infectious diseases such as Ebola and SARS — will be tested by a medical team from Beijing-based China-Japan Friendship Hospital, a hospital spokeswoman told Bloomberg News. The trial will be conducted in the central Chinese city of Wuhan, the epicenter of the outbreak that has sickened more than 17,000 people and killed more than 360 in China. Researchers will recruit up to 270 patients with mild and moderate pneumonia caused by the virus, according to Chinese news outlet The Paper, Bloomberg News reported.
Remdesivir is not approved for use by any drug regulator in the world, but it is being given to patients infected with the new coronavirus because there are no approved treatments, drug maker Gilead said in a statement. The company said it is working with Chinese health officials to organize the clinical trial to determine its effectiveness and safety of the drug in patients infected with the new coronavirus. The HIV medication Kaletra has also been recommended by China’s health regulator as an antiviral treatment for the new coronavirus, and clinical trials of that drug are also being arranged, according to The Paper.
On Sunday, officials reported three more cases of the new coronavirus in California, bringing the total in the United States to 11. Worldwide, there are now 146 coronavirus cases in at least 23 countries outside China, according to the World Health Organization. One death outside China has been reported in the Philippines.
Bloomberg News Article
More Information: CDC
Copyright © 2020 HealthDay. All rights reserved.