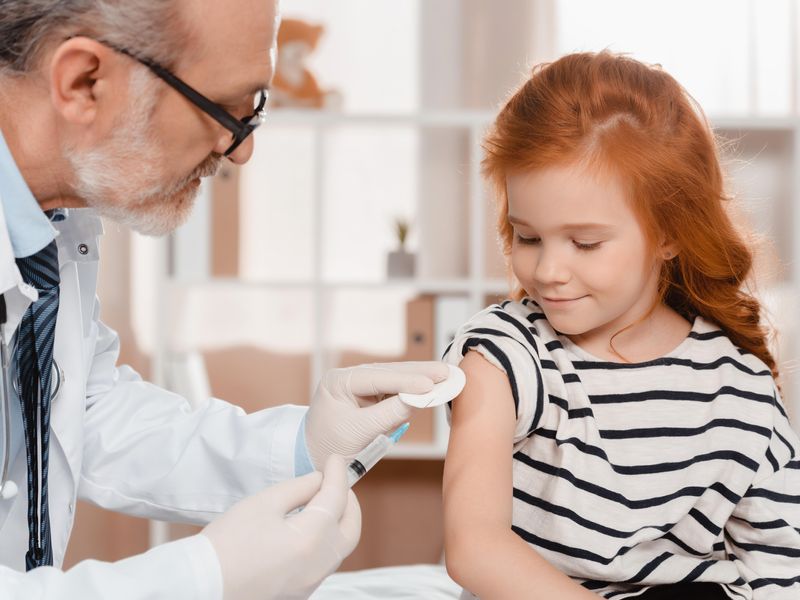
PCV15 includes two serotypes in addition to those in the PCV13 vaccine, and is recommended according to PCV13 dosing and schedules
THURSDAY, Sept. 15, 2022 (HealthDay News) — The 15-valent pneumococcal conjugate vaccine (PCV15) can be used for children aged younger than 19 years, according to recommendations issued by the Advisory Committee on Immunization Practices and published in the Sept. 16 issue of the U.S. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report.
Noting that PCV15 was licensed for use in adults aged 18 years and older in 2021, Miwako Kobayashi, M.D., Ph.D., from the CDC in Atlanta, and colleagues discuss the use of PCV15 for children.
The authors note that based on studies comparing antibody responses to PCV15 with those with PCV13, the U.S. Food and Drug Administration approved expanded use of PCV15 on June 17, 2022, to include persons aged 6 weeks to 17 years. In addition to the PCV13 serotypes, PCV15 contains serotypes 22F and 33F conjugated to CRM197. On June 22, 2022, the CDC Advisory Committee on Immunization Practices recommended PCV15 as an option for pneumococcal conjugate vaccination of persons aged younger than 19 years according to the PCV13 dosing and schedules currently recommended. No change was noted in the risk-based recommendations on use of PPSV23 for persons aged 2 to 18 years with certain underlying medical conditions that increase pneumococcal disease risk.
“PCV15 as an option for pneumococcal conjugate vaccination is expected to reduce pneumococcal disease incidence in children because it induces immunity against additional disease-causing serotypes,” the authors write.
Copyright © 2022 HealthDay. All rights reserved.