Tag: Food & Drug Administration
FDA OKs Device to Help Prevent Procedure-Related Stroke
Transcarotid system designed to help prevent stroke during stent and angioplasty procedures
FDA Expands Approval of Lucentis for Diabetic Retinopathy
To treat diabetic retinopathy in patients with diabetic macular edema
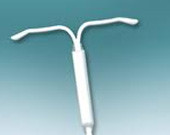
Implant, Hormonal IUD Effective Beyond FDA-Approved Duration
Etonogestrel implant, levonorgestrel IUD effective beyond three and five years, respectively
FDA Approves Ibrance to Treat Advanced Breast Cancer
For postmenopausal women with ER+, HER2− metastatic breast cancer
FDA Approves Internal Tissue Adhesive
TissuGlu connects tissue flaps stemming from surgery to remove excess fat, skin from stomach
FDA: Vyvanse Approved for Binge-Eating Disorder
Patients taking Vyvanse experienced a decrease in the number of binge eating days per week
FDA to Strengthen Approval Process for AEDs
Agency has received 72,000 reports of automated external defibrillators failing
FDA Approves Generic Form of Nexium
To treat gastroesophageal reflux disease in adults and children ages 1 and older
FDA Approves New Meningococcal Disease Vaccine
Bexsero is the second vaccine approved in the past three months to prevent this disease
Natpara OK’d to Treat Low Blood Calcium in Hypoparathyroidism
Medication recommended for use in patients for whom the potential benefits outweigh potential risk