Tag: Food & Drug Administration
Promacta Approval Expanded for Children With Chronic ITP
Builds on recent approval for ages 6 years and up for chronic immune thrombocytopenic purpura
FDA Approves Libido Pill for Women
Addyi to treat generalized hypoactive sexual desire disorder among premenopausal women
FDA Reveals More Violations by Medical Scope Maker
Infections in patients who underwent procedures with Olympus scopes in 2012 went unreported
FDA Approves First 3D Printed Prescription Medication
Epilepsy drug Spritam may be one of many custom-made medicines to come that will use the technology
FDA Approves Balloon Device to Treat Obesity
Inflated in stomach, it may create sense of fullness
FDA Approves Praluent for Certain Cases of High Cholesterol
First in a new class of injectable cholesterol-lowering medications
Delays Noted in the Reporting of Serious Patient Harms to FDA
Analysis found roughly 10 percent of cases were filed after 15-day deadline passed
FDA Approves Test to Differentiate HIV Viruses
Among people two years and older
FDA Approves Technivie for Hepatitis C
For people with chronic hepatitis C genotype 4
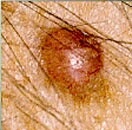
FDA Approves Odomzo for Recurring Basal Cell Carcinoma
Medication is designed to inhibit the Hedgehog pathway