Findings seen for maintenance therapy in patients with advanced or recurrent disease following response to systemic therapy
By Lori Solomon HealthDay Reporter
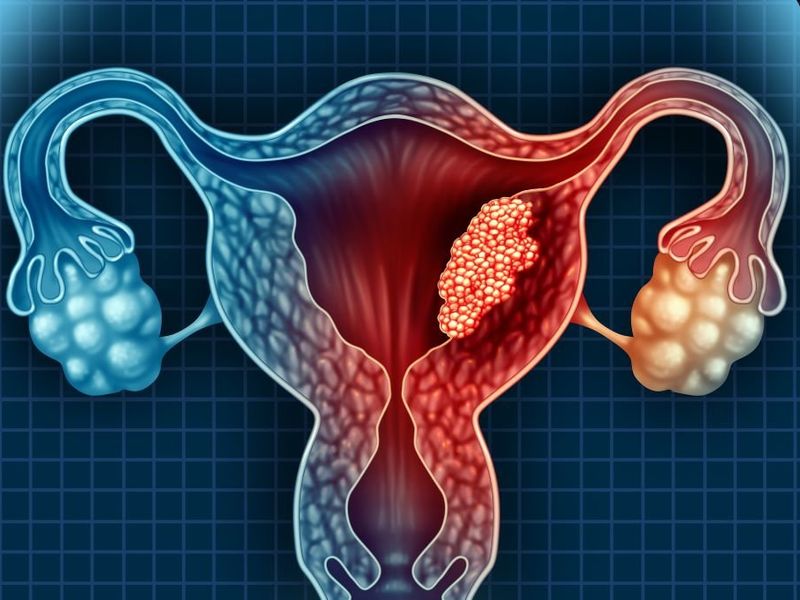
FRIDAY, July 28, 2023 (HealthDay News) — Maintenance therapy with selinexor provides durable survival benefits for endometrial cancer with TP53 mutations, according to a study presented during the July 2023 session of the American Society for Clinical Oncology Plenary Series.
Brian M. Slomovitz, M.D., from the Mount Sinai Medical Center in Miami Beach, Florida, and colleagues conducted the SIENDO phase 3 double-blind study evaluating selinexor versus placebo as maintenance therapy among 113 patients with advanced or recurrent endometrial cancer following response to prior systemic therapy and with wild-type TP53 (TP53wt) mutations (selinexor: 77 patients; placebo: 36 patients).
The researchers found that at a median follow-up of 20.3 months, 26.3 percent of patients were still on selinexor and 22.9 percent were still on placebo treatment. In the TP53wt subgroup, median progression-free survival (PFS) was 20.8 months with selinexor versus 5.2 months with placebo. Regardless of microsatellite stability status, efficacy was seen. The most common adverse events of any grade were nausea (90 percent with selinexor versus 34 percent with placebo), vomiting (61 versus 11 percent), and diarrhea (38 versus 34 percent). The most common grade 3+ adverse events included neutropenia (18 and 0 percent, respectively), nausea (12 and 0 percent, respectively), and thrombocytopenia (9 and 0 percent, respectively). Discontinuation due to treatment-emergent adverse events was reported in 15 and 0 percent of patients, respectively, in the two groups.
“This is an exciting step forward in potential treatment options that will be studied further,” Slomovitz said in a statement. “With a weekly oral dose of selinexor, there is a possibility to remain progression free for a much longer period than the current standard of care and substantially delay the initiation of second-line therapy.”
The study was funded by Karyopharm, the manufacturer of selinexor.
Copyright © 2023 HealthDay. All rights reserved.