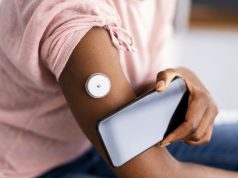
Many companies are illegally marketing smartwatch or smart ring devices that claim to measure blood glucose without piercing the skin
By Physician’s Briefing Staff HealthDay Reporter
THURSDAY, Feb. 22, 2024 (HealthDay News) — The U.S. Food and Drug Administration has issued an advisory regarding smartwatches and smart rings that claim to track blood glucose levels.
“Sellers of these smartwatches and smart rings claim their devices measure blood glucose levels without requiring people to prick their finger or pierce the skin. They claim to use noninvasive techniques,” the agency said. “These smartwatches and smart rings do not directly test blood glucose levels.” No such devices have ever been approved by the agency, and trusting them can be hazardous.
“For people with diabetes, inaccurate blood glucose measurements can lead to errors in diabetes management,” the agency warned. Those errors include taking the wrong doses of a drug that might send blood glucose plummeting to dangerous lows. In other cases, “taking too much of these medications can quickly lead to dangerously low glucose, leading to mental confusion, coma, or death within hours of the error,” the FDA warned.
The agency said consumers are able to buy these unapproved devices easily online. “These smartwatches and smart rings are manufactured by dozens of companies and sold under multiple brand names,” according to the FDA.
The FDA is asking the nation’s health care providers to warn patients of this danger. “This safety communication applies to any smartwatch or smart ring that claims to measure blood glucose without piercing the skin, regardless of manufacturer or brand,” the agency said.
The FDA added that it is working to prevent the illegal marketing of these devices throughout the United States.
Copyright © 2024 HealthDay. All rights reserved.