Abrysvo indicated to be given between 32 and 36 weeks of pregnancy to prevent lower respiratory tract disease caused by RSV in infants from birth through 6 months
By Physician’s Briefing Staff HealthDay Reporter
TUESDAY, Aug. 22, 2023 (HealthDay News) — Abrysvo received the first approval for a vaccine women can take during pregnancy to help protect their newborn from respiratory syncytial virus (RSV), the U.S. Food and Drug Administration announced Monday.
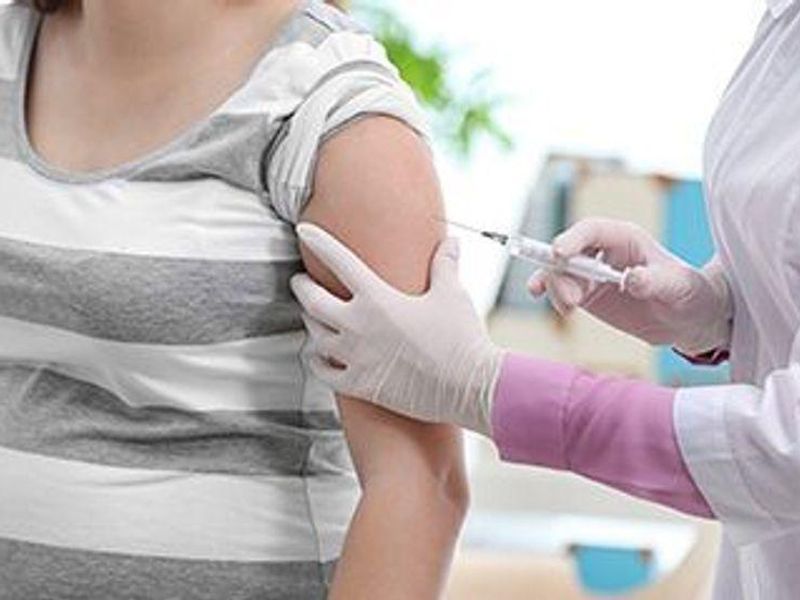
The vaccine is designed to be given to pregnant women between 32 and 36 weeks of pregnancy to prevent lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth through 6 months. Approval from the U.S. Centers for Disease Control and Prevention is still required before the vaccine is fully authorized for use. The vaccine is administered as a single-dose injection into the muscle. It is the same vaccine approved by the FDA and CDC in May to prevent RSV-caused illness in people ages 60 years and older.
“RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” Peter Marks, M.D., Ph.D., director of the FDA Center for Biologics Evaluation and Research, said in an agency statement. “This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
The vaccine’s safety and effectiveness were evaluated in a clinical study of about 3,500 pregnant women who received Abrysvo and 3,500 who received placebo. The vaccine reduced the risk for severe LRTD by 81.8 percent within three months after birth and by 69.4 percent within six months after birth. In a subgroup of pregnant people at 32 through 36 weeks of gestational age, of whom 1,500 received Abrysvo and 1,500 received placebo, the researchers found that Abrysvo reduced the risk for LRTD and severe LRTD by 34.7 and 91.1 percent, respectively, within three months after birth when compared with placebo. By 6 months of age, Abrysvo reduced the risk for LRTD and severe LRTD by 57.3 and 76.5 percent, respectively, the FDA noted.
Safety was evaluated in two studies, one in about 7,200 women, half of whom received Abrysvo, and the other in about 200 pregnant women, half of whom also received Abrysvo. Side effects included pain at the injection site, headache, muscle pain, and nausea. Preeclampsia occurred in 1.8 percent of pregnant individuals who received Abrysvo compared with 1.4 percent of those who received placebo, the FDA said. Low birth weight and jaundice also occurred at a higher rate in infants of pregnant recipients of Abrysvo versus placebo.
Prescribing information will include a warning about a “numerical imbalance” in preterm births in Abrysvo recipients: 5.7 percent versus 4.7 percent in those who received placebo, the FDA noted. The data are not sufficient to establish that the vaccine can cause preterm births but provides a warning to health care providers, the FDA said.
The approval was granted to Pfizer, which the FDA is requiring to conduct more studies to assess risks for preterm birth and preeclampsia.
Copyright © 2023 HealthDay. All rights reserved.