Young CA Survivors More Often Have Cost-Related Nonadherence
Risk of nonadherence was lower for survivors reporting a usual source of care than those without
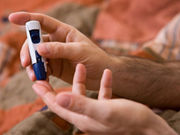
Sitagliptin Stimulates Distal Tubular Natriuresis in T2DM
Increase in intact plasma SDF-1α1-67 and decrease in truncated plasma SDF-1α3-67
Relapse Down With Clozapine, Injectables in Schizophrenia
Risk of rehospitalization substantially lower with long-acting injectables versus oral equivalents
Researchers Target Zolmitriptan Dosing for Pediatric Migraine
Study supports body-weight dosing of 5.0 mg and 2.5 mg for children >50 kg and <50 kg, respectively
Antidepressants in Pregnancy Don’t Affect Newborn Behavior
Newborns aren't more likely to be irritable, hard to feed, or sleepless, researchers say
SGLT2 Inhibitors Linked to Increased Risk of Ketoacidosis
Associated with approximately twice the risk of DKA compared to DPP4 inhibitors
Omalizumab Protects Against Early, Late Allergic Responses
Significant reduction in early allergic reaction after 4 weeks, with decrease in FEV1, exhaled nitric oxide
U.S. Leads in Income-Based Health Care Inequalities
More public tolerance for health care inequalities seen in United States than other countries
Lithium’s Risk to Fetus May Be Lower Than Previously Thought
Increased risk of cardiac defects seen, but magnitude of effect smaller than previously postulated
Severe Hypoglycemia Rates Have Equilibrated for DCCT Groups
Preceding episode of severe hypoglycemia deemed most powerful predictor of subsequent episodes