Voluntary Recall of Robaxin 750 mg Due to Dosage Misprint
Label should read 'two tablets three times daily,' not 'two to four tablets four times daily'
September 2018 Briefing – Pharmacy
Here are what the editors at HealthDay consider to be the most important developments in Pharmacy for September 2018. This roundup includes the latest...
Risk of Venous Thromboembolism Recurrence High
At 6 months, rates for provoked, unprovoked, cancer-related VTE are 6.8, 6.92, 9.06 per 100 person-years
Factors Associated With Phantom Odor Identified
NIH study shows 6.5 percent of U.S. adults aged 40 and up perceive phantom smells
More Non-Elderly Americans Uninsured in 2017 Versus 2016
More than 700,000 Americans uninsured in 2017 versus 2016, despite broad economic improvement
Intravitreal Triamcinolone Beats Periocular Tx for Macular Edema
Intravitreal triamcinolone acetonide, dexamethasone implant best for uveitic macular edema
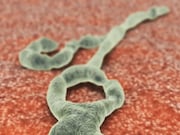
WHO: ‘Very High Risk’ That Ebola Will Spread From Congo
Agency says two cases of the deadly virus were confirmed near the border with Uganda
FDA Approves New Treatment for Squamous Cell Carcinoma
Libtayo is the sixth FDA-approved immune checkpoint inhibitor targeting the PD-1 pathway
FDA Approves New Drug for Antibiotic-Resistant Lung Disease
Arikayce approved to treat lung disease caused by Mycobacterium avium complex bacteria
Nintedanib Plus Sildenafil No Better Than Nintedanib in IPF
No benefit with sildenafil in idiopathic pulmonary fibrosis with severe impairment in gas exchange