Tramadol May Have Slight Effect on Reducing Chronic Pain
Evidence seen, however, for harmful effects from serious adverse events and several nonserious adverse events
Women’s Employment Trajectory Impacted by Menopause
Early natural menopause, surgical menopause both tied to leaving labor market
Spinal Manipulative Therapy for Low Back Pain Tied to Lower Risk for Opioid Use...
Findings seen for adult patients followed for two years compared with those prescribed ibuprofen for low back pain
FDA Approves At-Home Version of Lasix for Heart Failure Care
The device allows safe treatment for fluid buildup without hospital IV infusion
Majority of Patients in ED With Sickle Cell Crisis Not Triaged Appropriately
65.2 percent of patients with sickle cell disease with vaso-occlusive crisis assigned to emergency severity index 3
Congo’s War Cuts Off Medicine to Hundreds of Health Facilities
Shortages are leaving patients without treatment for malaria, HIV and other serious diseases
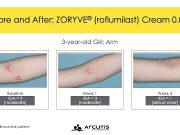
FDA Approves Zoryve for Atopic Dermatitis in Young Children
The once-daily cream can be used anywhere on the body and offers an alternative to steroids in children ages 2 to 5 years
Seizure Risk Up for Seniors in Nursing Homes Prescribed Tramadol and Antidepressants
Risk for seizures increased with concomitant use of tramadol with CYP2D6-inhibiting versus neutral antidepressants
Intranasal Adrenaline Offers Viable, Needle-Free Option for Anaphylaxis
Intranasal adrenaline demonstrated comparable or faster absorption compared with intramuscular treatment
Sotatercept Reduces Risk for Clinical Worsening in Adults With Recently Diagnosed PAH
Benefit with sotatercept seen among adults with pulmonary arterial hypertension diagnosed less than a year earlier