Tag: Side Effects
Systemic Reactogenicity Up With Heterologous COVID-19 Vaccine Doses
More reported feverishness, chills, fatigue, headache, joint pain, malaise, muscle ache after heterologous versus homogenous doses
ACC: Common Medications Can Raise Blood Pressure
Nearly one in five adults with hypertension take medications that can increase blood pressure, such as antidepressants, NSAIDs, oral steroids
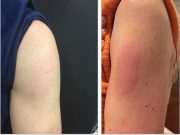
Delayed Hypersensitivity Reaction to Moderna COVID-19 Vaccine ID’d
Reaction occurred at or near injection site; reactions were pruritic, painful, and edematous pink plaques
Guidance Issued for Managing CVST After COVID-19 Vaccination
CVST with thrombocytopenia reported after COVID-19 vaccine with adenoviral vector; management similar to heparin-induced thrombocytopenia
12 Cases of CVST Reported for Ad26.COV2.S Vaccine in the U.S.
Time from vaccination to symptom onset varied from six to 15 days; initial presentation was headache in 11 cases
Side Effects of BNT162b2, ChAdOx1 nCoV-19 Vaccines Quantified
Systemic side effects for 13.5 and 22.0 percent after first, second dose of BNT162b2; 33.7 percent after first dose of ChAdOx1 nCoV-19
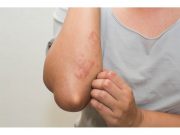
Cutaneous AEs Linked to Immune Checkpoint Inhibitor Therapy
Cutaneous immune-related adverse events seen in 25 percent of cancer patients initiating ICI therapy with median onset time of 113 days
Chronic Adverse Effects From Anti-PD-1 Immunotherapies Common
About 43 percent of patients who receive anti-PD1 therapy for advanced melanoma have chronic immune-related adverse effects
Many Older Adults Unaware of Potential Side Effects for New Rx
Physicians should spend more time discussing possible side effects of a newly prescribed medication during the office visit, authors say
AstraZeneca Vaccine Safe and Effective, European Medicines Agency Says
Officials hope announcement will ease concerns about possible rare side effects involving blood clots