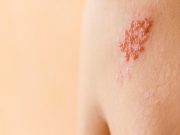
Tag: Herpes Zoster (Shingles)
Herpes Zoster Not Linked to Increased Dementia Risk
Small reduction in risk for dementia seen after the first year for people with zoster versus general population comparators
Incidence of Herpes Zoster Up for ≥50s With COVID-19 Diagnosis
Increased herpes zoster risk found for those with versus without COVID-19, with more pronounced risk following COVID-19 hospitalization
Recommendations Updated for Recombinant Zoster Vaccine
Two doses of RZV recommended for adults 19 years and older who are immunocompromised due to disease or therapy
Concurrent Zoster, Flu Vaccines Tied to Lower Subsequent Flu Vaccine Uptake
Patients may misattribute adverse effects commonly caused by the zoster vaccine to the influenza vaccine
Live Attenuated Zoster Vaccine Safe for Patients Receiving TNF Inhibitors
No cases of varicella infection found; cumulative incidence of varicella infection or shingles was 0 percent
2008 to 2018 Saw Increase in Shingles Vaccination in Over 60s
Women equally as likely as men to have received vaccine; coverage highest for non-poor, those with higher education
ASA: Herpes Zoster Vaccine May Reduce Risk for Stroke
Risk significantly reduced for stroke events, acute ischemic strokes, hemorrhagic strokes in older adults
Adult Exposure to Chickenpox Cuts Shingles Risk
A modest but long-lasting protective effect was observed, but full protection not achieved
Two-Dose Course of Vaccine After HSCT Cuts Incidence of Zoster
Incidence of herpes zoster decreased during median 21-month follow-up after autologous HSCT
2003 to 2014 Saw Incidence of Herpes Zoster Drop in Children
Vaccinated children had 78 percent lower rate of HZ incidence than unvaccinated children