Tag: Drug Approvals
FDA Approves Blenrep for Relapsed or Refractory Multiple Myeloma
Triplet combination treatment shows 51 percent reduction in the risk for death and tripled median progression-free survival versus a daratumumab-based triplet
FDA Approves Tezspire for Chronic Rhinosinusitis With Nasal Polyps
Approval is for add-on maintenance treatment in adults and pediatric patients ages 12 years and older
New Nonhormonal Drug Approved to Treat Menopause Symptoms
The once-daily pill elinzanetant is expected to be available within weeks
FDA to Fast-Track 9 Experimental Drugs Under New Trump Program
The drugs include potential new treatments for vaping addiction, deafness, pancreatic cancer and other serious health conditions
FDA Approves Jascayd for Idiopathic Pulmonary Fibrosis
Twice-daily oral tablet marks first approval of idiopathic pulmonary fibrosis in more than 10 years
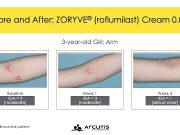
FDA Approves Zoryve for Atopic Dermatitis in Young Children
The once-daily cream can be used anywhere on the body and offers an alternative to steroids in children ages 2 to 5 years
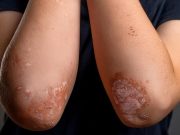
FDA Approves Tremfya for Pediatric Plaque Psoriasis, Active Psoriatic Arthritis
40 percent of children achieved complete skin clearance at week 16 with Tremfya versus placebo
FDA Approves Inluriyo for Advanced Breast Cancer
Approval is for adults with ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer
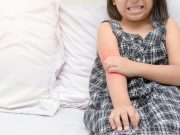
FDA Approves Opzelura for Atopic Dermatitis in Children
It is the first topical JAK inhibitor approved in the U.S. for pediatric atopic dermatitis
FDA Grants Accelerated Approval for First Treatment for Barth Syndrome
Forzinity helps improve strength of muscle to straighten the leg and works by improving mitochondria structure and function