Home 2015
Archives
CDC: Low-Income Southerners at Highest Risk of Vision Loss
Restricted access to eye and health care likely to blame, study authors suggest
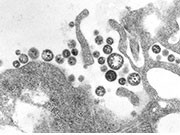
CDC: U.S. Traveler Returning From Liberia Dies of Lassa Fever
Only sixth known case in United States since 1969, but virus is much less deadly than Ebola
AMA: Avoiding Distress in Medical School
Drivers of distress include unsupportive learning environment, mistreatment, debt
CDC: Smoking Rates on Decline in Many States
But some people are using alternative tobacco products in addition to cigarettes
Large Practices Focused on Small Selection of EHR Products
Sixty percent of responses from clinicians in large practices were from users of 10 EHR products
CDC: Raw Tuna Suspected As Salmonella Source in Outbreak
At least 53 people in nine states have fallen ill, while 10 have been hospitalized, agency reports
Anxiety Reported by More Than 4 Million Working Americans
Data from SAMHSA's annual National Survey on Drug Use and Health 2008 to 2012
CDC Warns Against ‘Dangerous Breath Holding’ in Water
Even seasoned athletes are drowning because of dangerous underwater behaviors, agency says
More Evidence That C-Sections Should Be Avoided When Possible
More complications and lower chances of vaginal delivery for subsequent births
Washington D.C. Nabs Highest American Fitness Index Ranking
Followed by Minneapolis-St. Paul and San Diego as places that encourage exercise