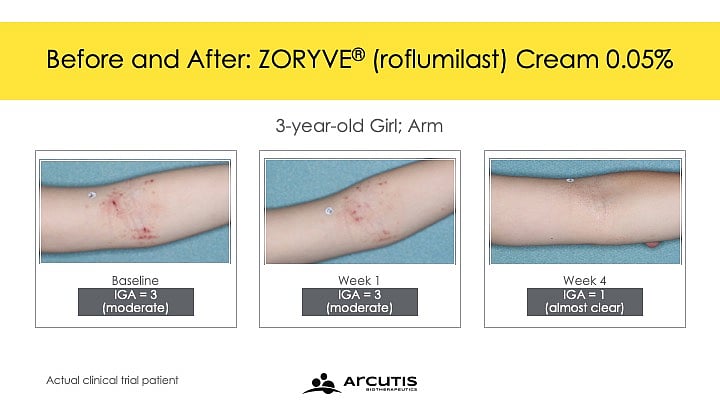
The once-daily cream can be used anywhere on the body and offers an alternative to steroids in children ages 2 to 5 years
By Lori Solomon HealthDay Reporter
THURSDAY, Oct. 9, 2025 (HealthDay News) — The U.S. Food and Drug Administration has approved the supplemental new drug application for Zoryve (roflumilast) cream 0.05 percent for the topical treatment of mild-to-moderate atopic dermatitis in children 2 to 5 years of age.
The once-daily cream can be used anywhere on the body and for any duration. This approval offers an alternative to steroids.
The approval is based on clinical trials that showed Zoryve cream rapidly improved the severity and extent of eczema signs and symptoms, with approximately 40 percent of children achieving a 75 percent improvement from baseline on the Eczema Area and Severity Index. Additionally, more than one-third of participants (35 percent) achieved clinically meaningful improvement in itch intensity in four weeks.
“Young children are particularly vulnerable to the bothersome symptoms of atopic dermatitis, because their immune system and skin barrier are less developed than those of older children and adults,” Korey Capozza, founder and executive director at Global Parents of Eczema Research, said in a statement. “This condition doesn’t just affect the child’s skin — it can affect the whole family by causing sleep disruption, emotional distress, and social isolation.”
Approval of Zoryve was granted to Arcutis Biotherapeutics.
Copyright © 2025 HealthDay. All rights reserved.