First and only drug releasing system is indicated for patients with BCG-unresponsive non-muscle-invasive bladder cancer seeking bladder preservation
By Lori Solomon HealthDay Reporter
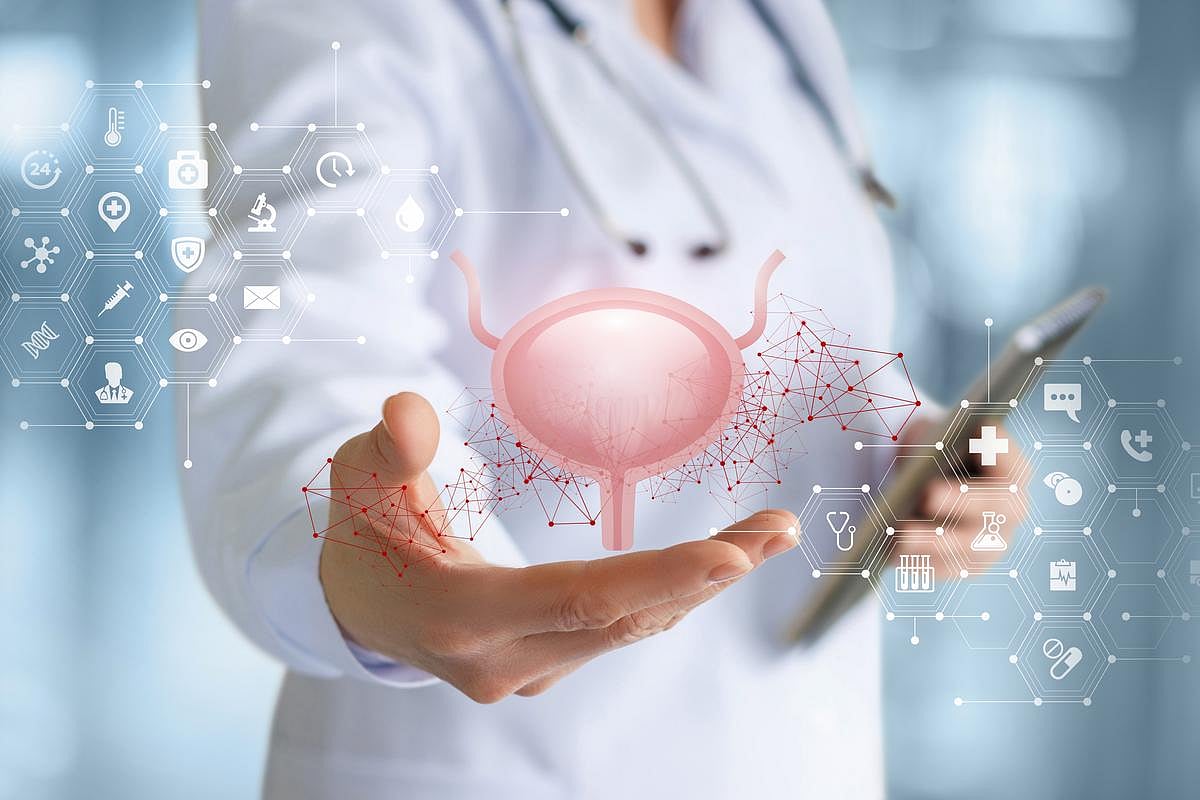
THURSDAY, Sept. 11, 2025 (HealthDay News) — The U.S. Food and Drug Administration has approved Inlexzo (gemcitabine intravesical system) for the treatment of patients with certain types of bladder cancer.
Specifically, Inlexzo is indicated for the treatment of adult patients with Bacillus Calmette-Guérin (BCG)-unresponsive, non-muscle-invasive bladder cancer with carcinoma in situ, with or without papillary tumors. The novel approach is designed for patients seeking bladder preservation and is the first and only intravesical drug-releasing system to provide extended local delivery.
The approval was based on data from the SunRISe-1 single arm, open-label phase 2b clinical study. More than eight in 10 patients with BCG-unresponsive non-muscle-invasive bladder cancer treated with Inlexzo achieved a complete response (82 percent), with strong durability. Just over half of patients with a complete response maintained a complete response for at least one year (51 percent).
“I see many patients that ultimately become BCG-unresponsive and often face life-altering bladder removal,” SunRISe-1 principal investigator Sia Daneshmand, M.D., from the University of Southern California in Los Angeles, said in a statement. “In my experience, Inlexzo is well tolerated and delivers clinically meaningful results. This will change the way we treat appropriate patients that haven’t responded to traditional therapy.”
Approval of Inlexzo was granted to Johnson & Johnson.
Copyright © 2025 HealthDay. All rights reserved.