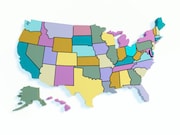
Medicaid Expansion Approved in Three Republican-Leaning States
Voters supported expansion in Utah, Nebraska, and Idaho
Reduced Survival for Patients With Idiopathic Parkinsonism
Mild cognitive impairment, freezing of gait, elevated leukocytes in CSF tied to shorter survival
Fluorescence Can ID High-Grade Glioma During Sx for Brain Tumor
Visible fluorescence shows presence of high-grade glioma cells within tumor mass during surgery
CDC: Cases of Polio-Like Illness Still Increasing in the U.S.
219 possible cases of acute flaccid myelitis reported in 25 states
Older Paternal Age Linked to Adverse Perinatal Outcomes
Higher risk for premature birth, low birth weight, low Apgar score seen with older paternal age
Removing Appendix May Lower Risk for Parkinson’s Disease
Healthy human appendix contains intraneuronal α-synuclein aggregates, truncation products
Abnormalities in Genes Linked to IRSP in Alzheimer’s Disease
Abnormalities, reductions in gene expression associated with insulin receptor signaling pathway
Surgery Restores Boy’s Ability to Walk Post-Acute Flaccid Myelitis
Nerve transfer surgery was performed on patient's leg, allowing him to walk
Most Meds Affecting Neurotransmitters Not Linked to Autism Risk
Prenatal exposure to 5 groups of meds had nominally significant links to ASD
Higher Serum Cortisol Level Linked to Lower Brain Volumes
Higher cortisol also associated with worse memory and visual perception in young, middle-aged adults