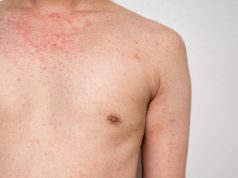
Measles Outbreak Spreads in Arizona-Utah Border Communities
Utah has reported 27 cases so far; Arizona has 42 confirmed cases, including one child who has been hospitalized
Illinois Confirms First Case of Rare Tick-Borne Powassan Virus
In severe cases, the virus may cause brain inflammation or meningitis
Pre-K and Elementary School Students Have Highest Virus Detection Rates
More than 80 percent of pre-K through 12th-grade students and staff have respiratory virus, acute respiratory illness episodes
Costco Recalls Ahi Tuna Poke in 30+ States Over Listeria Risk
No illnesses have been reported so far, but officials warn the product should not be eaten
Inpatient Hep C Consultation After Delivery Tied to Better Treatment Completion
Findings compared with traditional postpartum outpatient referral for women with HCV-affected pregnancies
Circulating Tumor HPV DNA Can ID HPV+OPSCC Years Before Diagnosis
Sensitivity of detection increased to 96 percent; maximum lead time increased to 10.3 years with application of machine learning model
New Vaccine Panel Recommends Doctor Consults Before COVID Shots
Guidance may limit pharmacy access, where most Americans got last year’s vaccine
Rare Flesh-Eating Bacteria Claims Fifth Life in Louisiana
Cases are on the rise across the region; state has already confirmed 26 cases this year
West Coast States Issue Their Own COVID, Flu, RSV Vaccine Rules
California, Hawaii, Oregon and Washington recommend COVID shots for young children, older adults and pregnant women
CDC Advisers Limit MMRV Combo Vaccine, Delay Hepatitis B Vote
The panel’s vote sparked criticism from many pediatricians and health leaders