Two Case Series Describe Acute Pediatric Hepatitis of Unknown Cause
Unclear whether human adenovirus is the causative agent for acute hepatitis in these children
CDC Backs COVID-19 Vaccine From Novavax
Vaccine uses nanoparticles made of proteins from the surface of the coronavirus to stimulate an immune response
Risks for Diabetes, CVD Up in Acute, Postacute COVID-19 Phases
However, from 13 to 52 weeks after COVID-19, no increase was seen for diabetes mellitus, cardiovascular disease
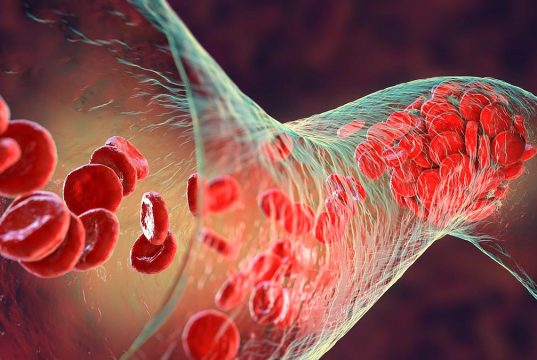
Increased Blood Viscosity Linked to Mortality in COVID-19
Hospitalized COVID-19 patients with increased estimated blood viscosity may have greater risk for in-hospital mortality
CDC Stops Tracking COVID-19 Cases on Cruise Ships
Decision allows cruise lines to set their own COVID-19 policies
Fauci to Retire by End of Biden’s Term
Fauci has been a government scientist for 50 years serving under seven presidents
Fewer HIV Tests, Cases ID’d in Children at Start of Pandemic
From October 2019 to September 2020, 21 percent decrease seen in outpatient department testing, increases observed in other testing strategies
Fourth COVID-19 Vaccine Dose Safe for Immunocompromised
Local and systemic reactions reported less frequently after fourth dose versus dose 3 of primary vaccination series
U.S. Extends COVID-19 Public Health Emergency Another Three Months
Separate emergency declaration allows for the emergency use authorization of testing, treatments, and vaccines
More Hospitalizations, Illnesses Reported From Daily Harvest Crumbles
Company said last week that it is doing comprehensive testing to rule out potential causes