Limited English Proficiency Tied to Worse Access to Health Care
Adults with limited English proficiency more likely to lack usual source of care, be overdue for receipt of preventive services
In COVID ‘Rebound,’ Biden Tests Positive for Second Day in a Row
Despite the test results, the president 'has experienced no reemergence of symptoms and continues to feel quite well'
USDA Gets Tough on Salmonella in Breaded Chicken Products
A very low level of Salmonella contamination in the products would lead to regulatory action
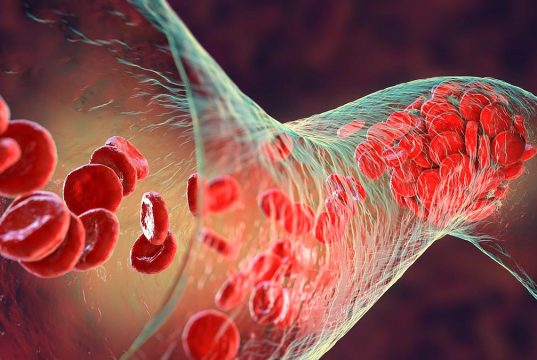
Vaccination Cuts Risk for Myocardial Infarction, Stroke After COVID-19
Findings show lower hospitalization risk 31 to 120 days after COVID-19 infection
Smoking Tied to Higher Risk for Severe COVID-19
Higher risks for death, mechanical ventilation, and major cardiovascular event all independent of sociodemographic factors and medical history
Reformulated Booster Shots Ready by September, White House Says
Biden administration is working on contracts for the reformulated booster doses
San Francisco, N.Y. State Declare Monkeypox Public Health Emergencies
Declaring emergency will expand testing and vaccines and apply pressure to the federal government to take the outbreak more seriously
Theophylline Nasal Irrigation Studied in COVID-19-Related Smell Loss
In phase 2 trial, subjective assessments suggest improvement in COVID-19-related olfactory dysfunction, but study results were inconclusive
COVID-19 Booster May Spur Seroconversion in Blood Cancer Patients
Among nonresponders to initial COVID-19 vaccination, seroconversion occurred in 56 percent following booster dose
Rate of In-Hospital Adverse Events Declined From 2010 to 2019
Adverse event rates decreased for patients admitted for acute myocardial infarction, heart failure, pneumonia, and major surgical procedures