Boy Dies From Brain-Eating Amoeba After Swimming in S.C. Lake
By I. Edwards HealthDay Reporter
MONDAY, July 28, 2025 (HealthDay News) — A 12-year-old South Carolina boy has died after being infected by a rare,...
Dozens Sick After Eating THC-Tainted Food at Wisconsin Pizzeria
By I. Edwards HealthDay Reporter
MONDAY, July 28, 2025 (HealthDay News) — A pizza shop in Wisconsin accidentally served food made with oil containing THC,...
Ritz Update: FDA Issues Risk Warning Due to Undeclared Peanuts in Some Cracker Sandwiches
By Denise Mann HealthDay Reporter
FRIDAY, July 25, 2025 (HealthDay News) — Ritz peanut butter cracker sandwiches are being recalled due to the possible presence of...
Tuna Recalled in Seven States Over Listeria Fears
By Denise Mann HealthDay Reporter
WEDNESDAY, July 23, 2025 (HealthDay News) — Two types of tuna sold in seven states are being recalled due to listeria concerns.The...
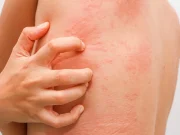
Study Compares Benefits, Harms of Treatments for Chronic Hives
Systematic review and network meta-analysis included 93 trials evaluating 42 interventions
More Than 110,000 Rich’s Ice Cream Bars Recalled Due to Listeria Risk
By Denise Mann HealthDay Reporter
WEDNESDAY, July 23, 2025 (HealthDay News) — More than 100,000 Rich's Ice Cream Co. bars have been recalled due to...
Millions of Backyard Pools Recalled After Drowning Deaths of Nine Children
By Denise Mann HealthDay Reporter
MONDAY, July 21, 2025 (HealthDay News) — Close to 5 million above-ground swimming pools have been recalled following the drowning...
FDA Elevates Dubai Chocolate Spread Recall to Class 1 Over Salmonella Risk
By Denise Mann HealthDay Reporter
MONDAY, July 21, 2025 (HealthDay News) — The U.S. Food and Drug Administration (FDA) has escalated the recall of a...
Urgent Care Visits Often Linked to Inappropriate Prescribing
Overall, 12.4, 9.1, and 1.3 percent of urgent care visits led to antibiotic, glucocorticoid, and opioid prescription fills, respectively
Oral Ondansetron Beneficial for Pediatric Gastroenteritis-Linked Vomiting
Lower risk for moderate-to-severe gastroenteritis seen during seven days after enrollment with oral ondansetron versus placebo