Doctors Need to Be Mindful of What They Post on Social Media
Investigators find many examples of unprofessional, 'potentially objectionable' behavior online
Platelet-Rich Plasma Safe, Effective for Skin Rejuvenation
Sustained improvement, patient satisfaction higher versus readymade growth factors
Tripeptide/Hexapeptide System Effective After Laser Resurfacing
Satisfaction up with tripeptide/hexapeptide topical system post fractionated CO2 laser resurfacing
Specific Factors Influence First-Line Biologic Rx for Psoriasis
Presence of psoriatic arthritis, weight, employment status, baseline disease severity all affect selection
$16 Billion Spent on Cosmetic Surgery by Americans in 2016
New report details costs of most popular plastic surgery procedures
Novel Laser Device Effective for Nasal Telangiectasia Treatment
Treatment effective in all patients, with most needing only one treatment session
Insecticide Resistance Thwarting Fight Against Bed Bugs
Chemicals alone won't vanquish the insects, researchers say
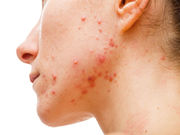
Mask With Myoinositol/Trehalose Aids Adult Female Acne
Mask contains an androgen inhibitor and trehalose-loaded liposomes
Optimal Features for Surgically Enhanced Lips Determined
Lower lip should be ~1.6 times the height of the upper lip and retain Cupid's bow
MACRA Changes Government Approach to Doctor Payment
New approach pays providers based on quality, value, and results delivered, but questions remain