Better event-free survival outcomes seen for durvalumab plus FLOT versus FLOT alone in phase 3 randomized trial
By Elana Gotkine HealthDay Reporter
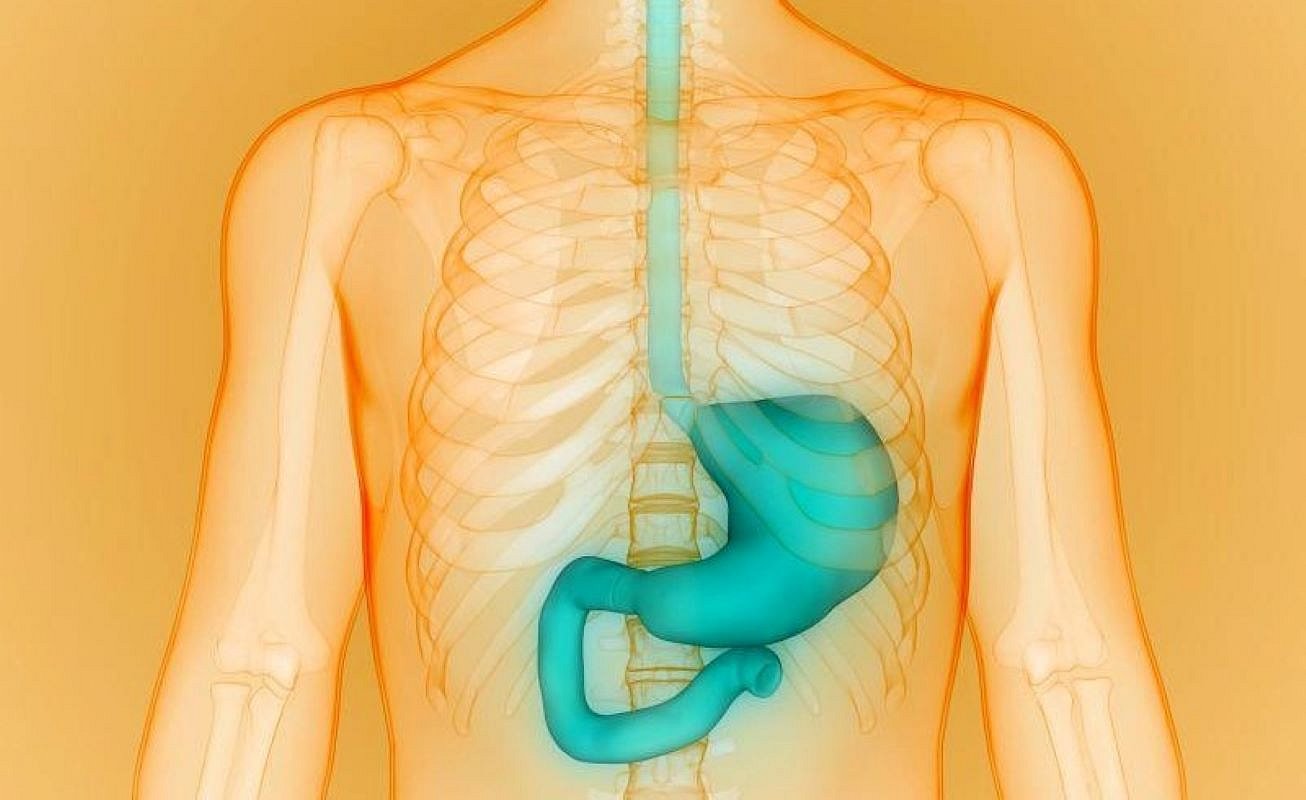
THURSDAY, June 5, 2025 (HealthDay News) — For patients with resectable gastric or gastroesophageal junction adenocarcinoma, durvalumab plus fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) yields better event-free survival outcomes than FLOT alone, according to a study published online June 1 in the New England Journal of Medicine. The research was published to coincide with the annual meeting of the American Society of Clinical Oncology, held from May 31 to June 4 in Chicago.
Yelena Y. Janjigian, M.D., from Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine in New York City, and colleagues conducted a phase 3, double-blind, randomized trial involving patients with resectable gastric or gastroesophageal junction adenocarcinoma. Participants were randomly assigned to receive durvalumab (1,500 mg) or placebo every four weeks plus FLOT for four cycles, followed by 10 cycles of durvalumab or placebo every four weeks (474 patients to each group). Event-free survival was the primary end point.
The researchers found that the two-year event-free survival was 67.4 and 58.5 percent among those in the durvalumab and placebo groups, respectively (hazard ratio for event or death: 0.71; 95 percent confidence interval, 0.58 to 0.86; P < 0.001). Two-year overall survival was 75.7 and 70.4 percent in the durvalumab and placebo groups, respectively (piecewise hazard ratio for death during months 0 to 12: 0.99; 95 percent confidence interval, 0.70 to 1.39; piecewise hazard ratio for death during the period from month 12 onward: 0.67; 95 percent confidence interval, 0.50 to 0.90; P = 0.03 by a stratified log-rank test [exceeding the significance threshold of P < 0.0001]). The percentage of patients with a pathological complete response was 19.2 and 7.2 percent in the durvalumab and placebo groups, respectively (relative risk: 2.69; 95 percent confidence interval, 1.86 to 3.90).
“This is a major step forward for patients facing this difficult diagnosis,” Janjigian said in a statement. “To be able to tell a patient that their cancer has completely responded to treatment — and that they are cured — is one of the most rewarding moments in oncology. These results bring us closer to making that outcome a reality for many more patients worldwide.”
Several authors disclosed ties to biopharmaceutical companies, including AstraZeneca, which manufactures durvalumab and funded the trial.
Copyright © 2025 HealthDay. All rights reserved.