Favorable bowel HRQOL seen with stereotactic body radiation therapy compared with intensity-modulated radiation therapy; no difference seen in disease-free survival
By Elana Gotkine HealthDay Reporter
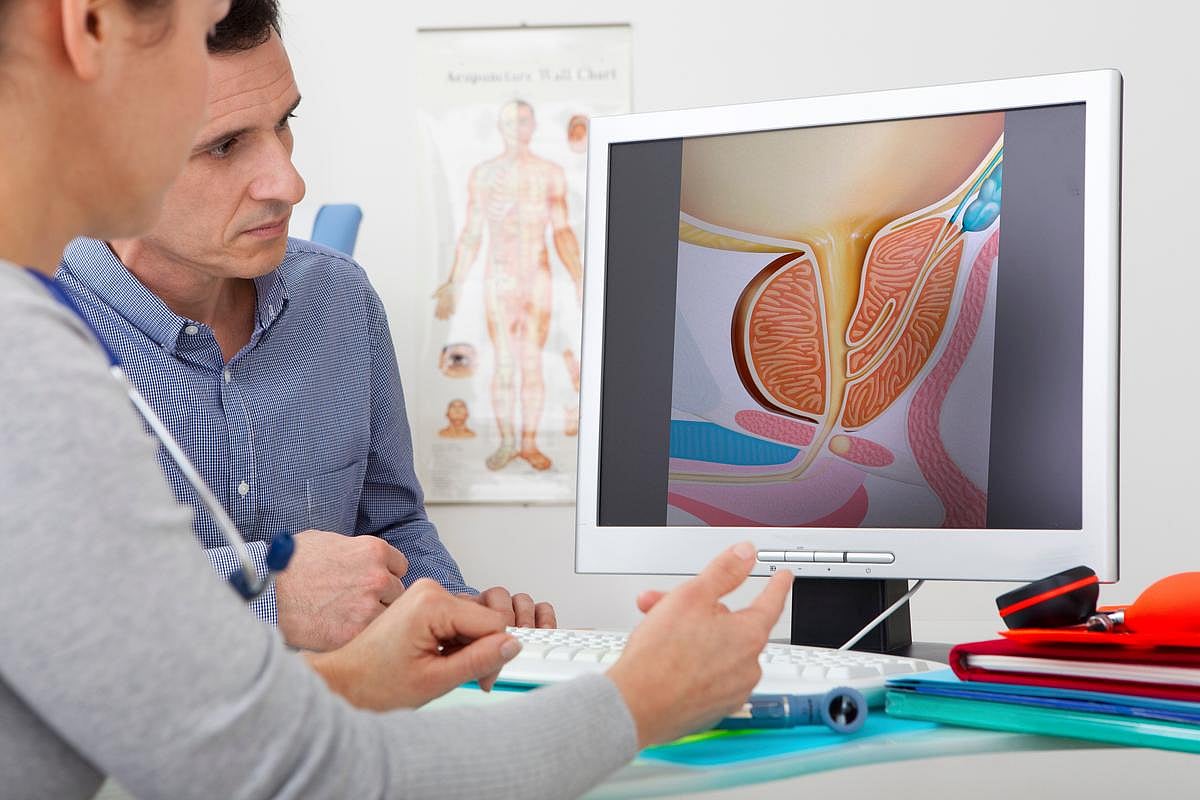
FRIDAY, Oct. 3, 2025 (HealthDay News) — For patients with intermediate-risk localized prostate cancer, stereotactic body radiation therapy (SBRT) yields favorable bowel health-related quality of life (HRQOL) compared with moderately hypofractionated intensity-modulated radiation therapy (M-IMRT), according to a study presented at the annual meeting of the American Society for Radiation Oncology, held from Sept. 27 to Oct. 1 in San Francisco.
Rodney J. Ellis, M.D., from the University South Florida in Tampa, and colleagues examined the superiority of disease-free survival (DFS) and patient-reported HRQOL among patients with intermediate-risk prostate cancer. Participants were randomly assigned to receive SBRT (36.25 Gy in five fractions; 353 patients) or IMRT (70 Gy in 28 fractions or 60 Gy in 20 fractions; 345 patients).
The researchers found that in the SBRT arm, significantly fewer patients experienced a two-year minimal clinically important decline (MCID) in the bowel domain of the HRQOL (34.9 versus 43.8 percent). No significant difference was seen between the groups in MCID frequency for the urinary irritation/obstruction domain (33.7 versus 34.7 percent). A significant treatment effect in favor of SBRT was seen in an analysis of the longitudinal bowel domain scores (least squares mean, 2.68). Use of a rectal spacer occurred in 56 and 55 percent of patients receiving SBRT and IMRT, respectively, resulting in superior Expanded Prostate Cancer Index Composite bowel domain scores (least squares mean, −2.81). For DFS, the futility bound was crossed in the interim analysis, indicating lack of superiority for SBRT over IMRT.
“The results help clarify what patients can expect from shorter versus longer courses of radiation therapy and enable more personalized treatment decisions based on individual priorities,” Ellis said in a statement.
Copyright © 2025 HealthDay. All rights reserved.