Findings seen for patients being treated for neovascular age-related macular degeneration or diabetic macular edema
By Lori Solomon HealthDay Reporter
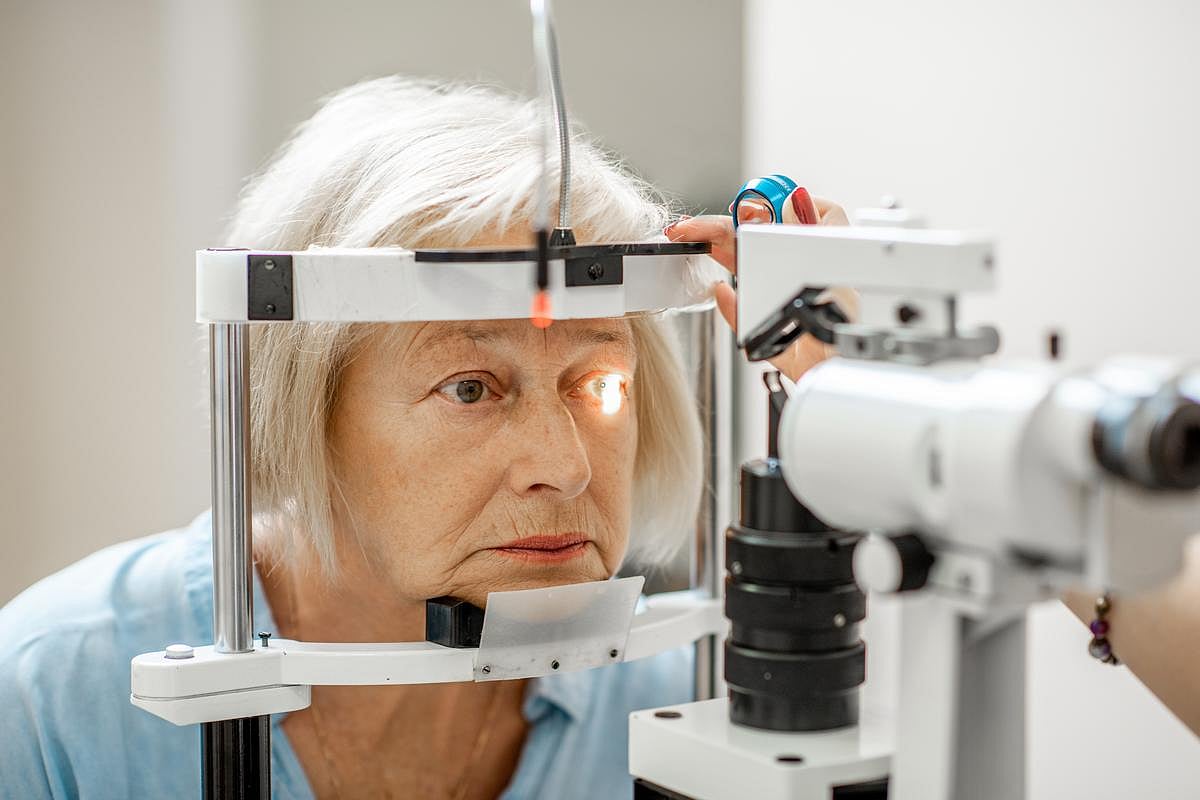
FRIDAY, May 9, 2025 (HealthDay News) — The incidence of mild intraocular inflammation (IOI) in the real world occurs more frequently after intravitreal injection of aflibercept than in clinical trials, according to a study published online May 1 in JAMA Ophthalmology.
Karoline E. Binder, M.D., from the Technical University of Munich, and colleagues assessed the incidence of IOI after intravitreal injection of aflibercept, 8 mg, in real-world practice. The analysis included 41 patients (23 with neovascular age-related macular degeneration and 18 with diabetic macular edema) who were treated with 136 intravitreal aflibercept, 8 mg, injections.
The researchers reported that five patients developed mild sterile IOI within one to three days after the intervention (incidence per injection, 3.7 percent; incidence per patient, 12 percent). Only one patient developed inflammation after the first dose, while the other four had prior exposure to aflibercept. All patients were treated with local anti-inflammatory therapy (topical or subconjunctival corticosteroids), while two patients received additional systemic oral corticosteroids. After IOI resolved, there was no reduction in best-corrected visual acuity.
“Although the IOI found in this series can be considered mild, careful evaluation of patient-associated risk factors and thorough patient education are warranted,” the authors write.
Several authors disclosed ties to the pharmaceutical industry.
Abstract/Full Text (subscription or payment may be required)
Copyright © 2025 HealthDay. All rights reserved.