Addition of revascularization to optimized medical therapy not beneficial in patients with carotid stenosis ≥50 percent and low or intermediate predicted stroke risk
By Elana Gotkine HealthDay Reporter
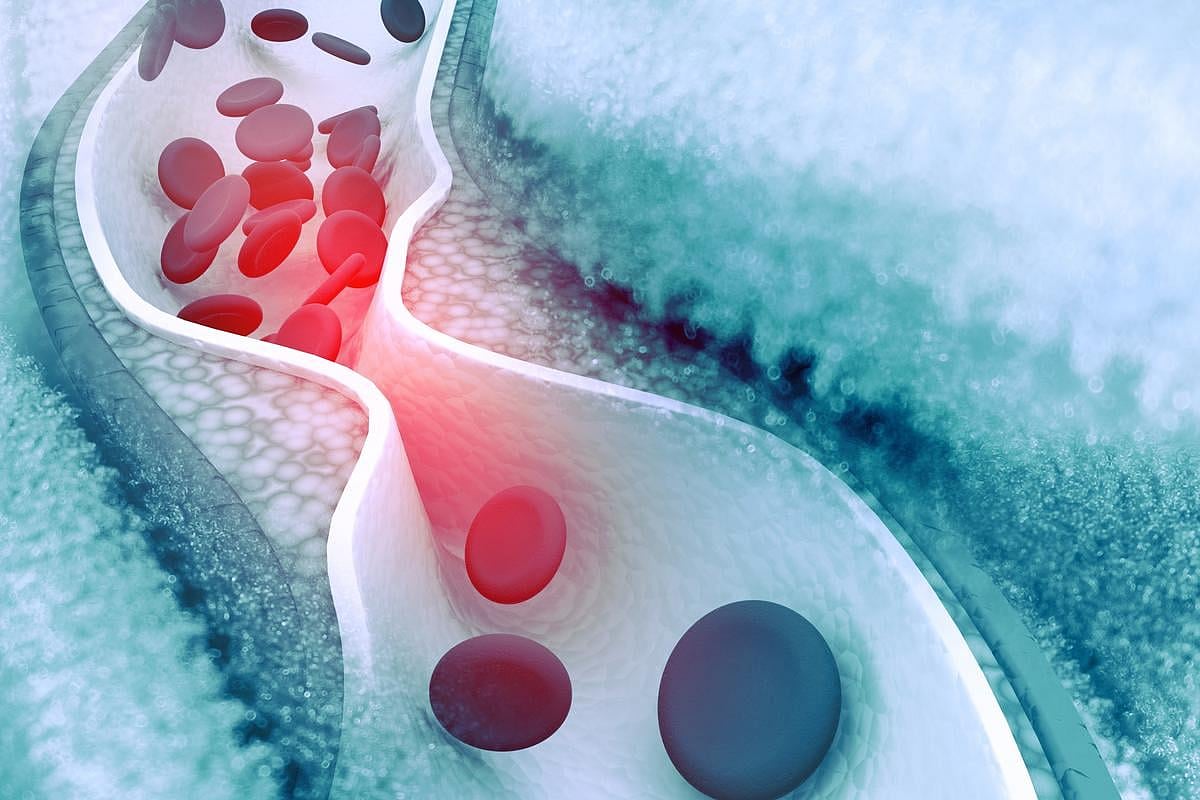
FRIDAY, May 2, 2025 (HealthDay News) — For patients with asymptomatic or symptomatic carotid stenosis of 50 percent or greater with a low or intermediate predicted stroke risk, there is no benefit for the addition of revascularization to optimized medical therapy (OMT) in the first two years, according to a study published in the May issue of The Lancet Neurology.
Simone J.A. Donners, M.D., from UMC Utrecht in the Netherlands, and colleagues examined whether patients with asymptomatic and symptomatic carotid stenosis with a low or intermediate predicted risk fir stroke, who received OMT, would benefit from additional revascularization in a multicenter randomized trial. Patients aged 18 years and older, with asymptomatic or symptomatic carotid stenosis of 50 percent or greater, and a five-year predicted risk for ipsilateral stroke of less than 20 percent, were randomly assigned to OMT alone or OMT plus revascularization (215 and 214 patients, respectively).
The researchers found that with respect to the primary hierarchical outcome composite of periprocedural death, fatal stroke, or fatal myocardial infarction; nonfatal stroke; nonfatal myocardial infarction; or new silent cerebral infarction on imaging assessed two years after randomization, no benefit was recorded in favor of either treatment group, with 11.4 and 11.3 percent wins for the OMT alone and OMT plus revascularization groups, and 77.3 percent ties between groups.
“We cannot rule out the possibility that revascularization will provide a small to moderate benefit in our patients beyond two years. We are, therefore, continuing follow-up to five years after randomization,” the authors write.
Several authors disclosed ties to the biotechnology, publishing, and medico-legal industries.
Editorial (subscription or payment may be required)
Copyright © 2025 HealthDay. All rights reserved.