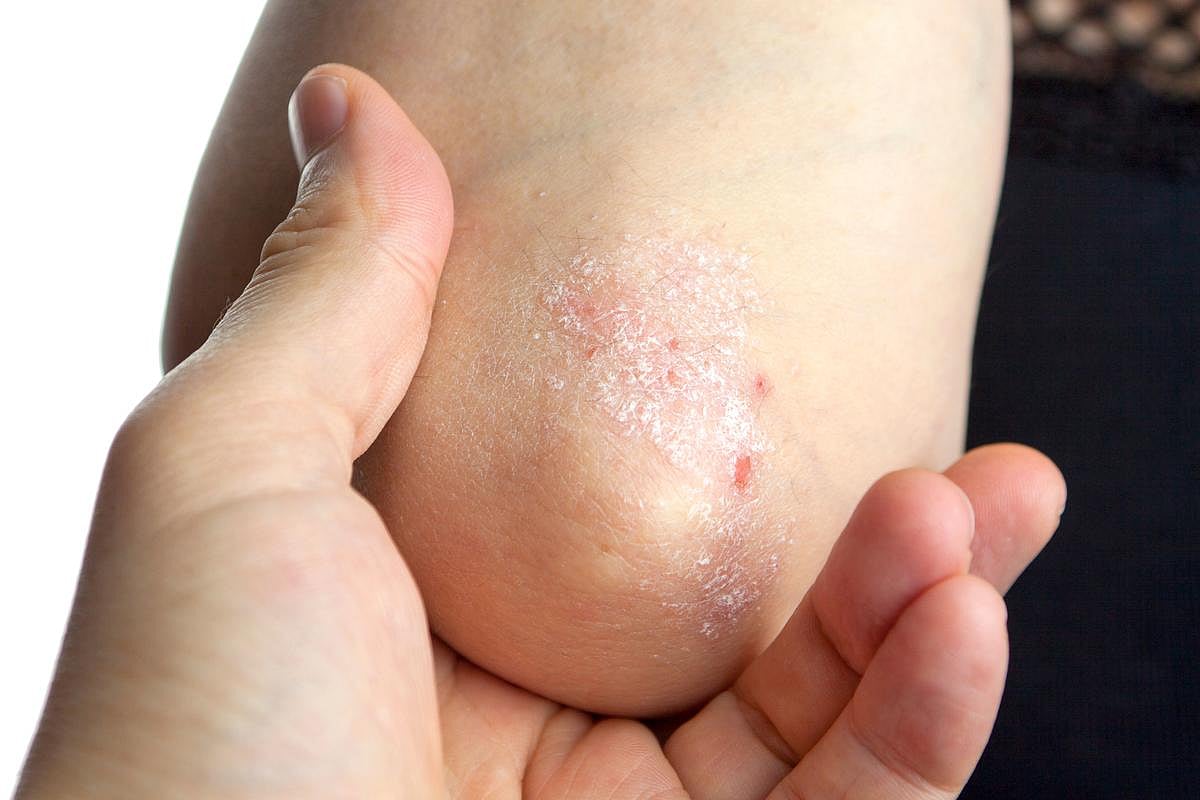
Icotronkinra results in significantly higher incidence of complete skin clearance at week 16 than placebo
By Elana Gotkine HealthDay Reporter
WEDNESDAY, Nov. 12, 2025 (HealthDay News) — For adults and adolescents with moderate-to-severe plaque psoriasis, selective blockade of the interleukin-23 receptor with the targeted oral peptide icotrokinra yields a significantly higher incidence of skin clearance at week 16 than placebo, according to a study published in the Nov. 6 issue of the New England Journal of Medicine.
Robert Bissonnette, M.D., from Innovaderm Research in Montreal, and colleagues conducted a phase 3, randomized trial involving adults and adolescents (aged 12 years and older) with moderate-to-severe plaque psoriasis. Participants were randomly assigned to receive either icotrokinra (200 mg once daily through week 24) or placebo through week 16 followed by transition to icotrokinra (456 and 228 patients, respectively).
The researchers found that 65 percent of the participants receiving icotrokinra and 8 percent of those receiving placebo had an Investigator’s Global Assessment (IGA) score of 0 or 1 with ≥2-point reduction from baseline at week 16, and 50 and 4 percent, respectively, had a ≥90 percent reduction from baseline in the Psoriasis Area and Severity Index (PASI) score. At week 16, complete clearance of skin was significantly more likely with icotrokinra than placebo (IGA score 0: 33 versus 1 percent; 100 percent reduction from baseline in the PASI score: 27 versus 1 percent). In each group, 49 percent of patients had at least one adverse event through week 16.
“Once-daily icotrokinra, a systemic targeted oral peptide binding to the interleukin-23 receptor, was effective for treating plaque psoriasis in adults and in adolescents, an age group with limited systemic treatment options,” the authors write.
The study was funded by Johnson & Johnson, the manufacturer of icotrokinra.
Editorial (subscription or payment may be required)
Copyright © 2025 HealthDay. All rights reserved.