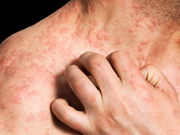
Four of 10 patients show response to treatment, with one patient experiencing complete response
THURSDAY, Dec. 29, 2016 (HealthDay News) — Ustekinumab is tolerated for treatment of atopic dermatitis (AD) in certain patients, according to a research letter published online Dec. 22 in the British Journal of Dermatology.
Eilis Nic Dhonncha, from the University Hospital Waterford in Ireland, and colleagues examined the clinical response to and tolerability of ustekinumab in treatment of severe AD in a single-center study involving 10 patients with AD. Ustekinumab was prescribed for off-label use as per the psoriasis schedule; patients received 45 or 90 mg ustekinumab subcutaneous injection at weeks zero, four, and every 12 weeks thereafter. The median duration of treatment was 10 months.
The researchers found that four of the 10 patients responded to treatment and six failed to respond. One patient achieved complete response requiring no topical steroid use, and three patients had almost complete response requiring only minimal amounts of intermittent topical steroids. Three of the patients received a higher dose of ustekinumab; one demonstrated almost complete response and two had no response to treatment. Two of the patients remained on ustekinumab at least two years after starting treatment, with excellent sustained disease control. Treatment failure was the most common reason for stopping ustekinumab (six patients).
“We report the largest retrospective case series to date outlining our experience with ustekinumab in the treatment of severe atopic dermatitis, and our study contributes to the growing anecdotal experience of ustekinumab in AD,” the authors write.
Full Text (subscription or payment may be required)
Copyright © 2016 HealthDay. All rights reserved.