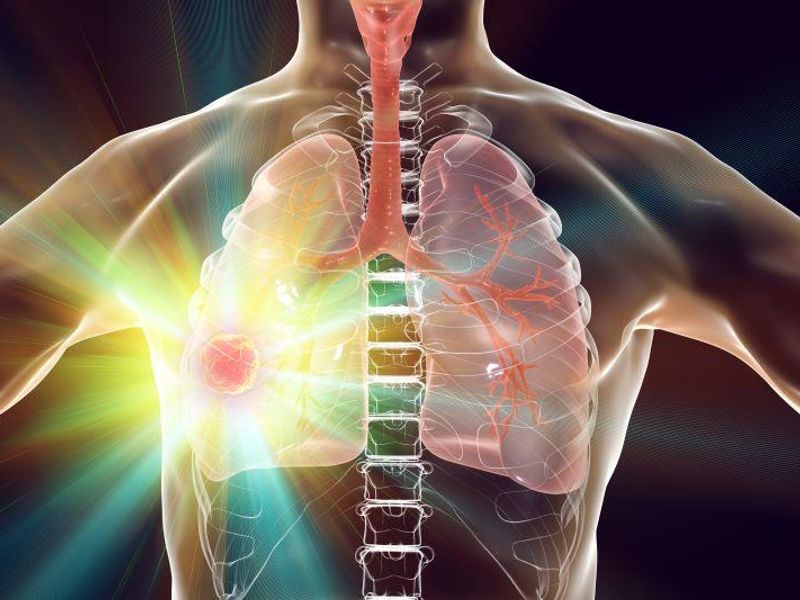
Eight of nine patients with major pathological response (MPR) were alive and disease-free at five years, while six of 11 without MPR experienced relapse
By Elana Gotkine HealthDay Reporter
WEDNESDAY, Feb. 15, 2023 (HealthDay News) — For patients with resectable non-small cell lung cancer (NSCLC), neoadjuvant nivolumab is associated with positive five-year outcomes, according to a study published online Feb. 15 in Clinical Cancer Research.
Samuel Rosner, M.D., from the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center in Baltimore, and colleagues present five-year clinical outcomes from a first phase I/II trial of neoadjuvant nivolumab in resectable NSCLC. Twenty-one patients with stage I to IIIA NSCLC were administered two doses of nivolumab for four weeks before surgery.
The researchers found that the five-year recurrence-free survival (RFS) and overall survival rates were 60 and 80 percent, respectively, with a median follow-up of 63 months. Trends toward favorable RFS were seen for the presence of major pathological response (MPR) and pretreatment tumor programmed death-ligand 1 positivity (hazard ratios, 0.61 [95 percent confidence interval, 0.15 to 2.44] and 0.36 [95 percent confidence interval, 0.07 to 1.85], respectively). Eight of nine patients with MPR were alive and disease-free at five-year follow-up; no cancer-related deaths were seen for those with MPR. However, six of 11 patients without MPR experienced relapse and three died.
“Neoadjuvant nivolumab monotherapy in NSCLC led to favorable long-term clinical outcomes, with a low rate of toxicity,” the authors write. “Clinicians should be confident in using immune checkpoint blockade in the preoperative setting.”
Several authors disclosed financial ties to pharmaceutical companies, including Bristol Myers Squibb, which manufactures nivolumab and partially funded the study.
Copyright © 2023 HealthDay. All rights reserved.